MEPhI scientists as part of an international scientific team have established that an organometallic frame structure based on tannic acid can effectively capture uranyl, the most dangerous component of liquid radioactive waste. The results of the study were published in the highly rated scientific journal Langmuir .
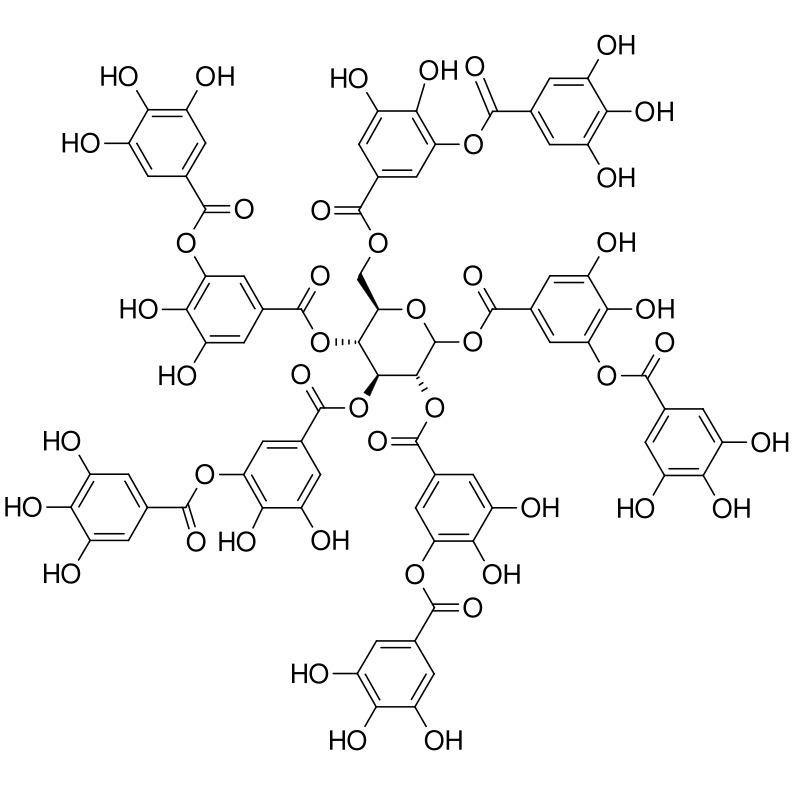
Molecular structure of tannic acid
Tannic acid is a well-known natural adsorbent; it is found in many plants and was used to treat poisoning until the beginning of the 20th century. Many hydroxyl groups make it "sticky" - able to adsorb different molecules due to hydrogen bonds. For the same reason, it is highly soluble in water, which is why so far it has not been possible to use it to treat liquid waste, said the author of the study, MEPhI professor Konstantin Katin.
“The shortcomings of a particular material are often a continuation of its merits. The adsorption properties of tannic acid are very attractive from the point of view of the purification of liquid radioactive waste. However, its high solubility made any developments in this direction unfeasible until we succeeded in synthesizing an organometallic framework based on tannic acid and iron. Such a framework is insoluble, but at the same time retains excellent adsorption properties,” he said.
Using quantum chemical calculations, the researchers theoretically predicted the structure and characteristics of an organometallic framework based on tannic acid and iron, and then tested them in practice. They conducted a series of experiments using infrared spectroscopy, scanning electron microscopy, X-ray analysis and adsorption studies and confirmed the ability of the organometallic framework structure to effectively capture uranyl, the most dangerous component of liquid radioactive waste.
Uranium is a valuable metal and an important raw material for nuclear energy, so its extraction from waste can be of not only environmental but also economic importance. A significant amount of uranium enters the water as a result of the operation of nuclear power plants, as well as from natural causes, such as volcanic eruptions.
“The complex we have synthesized is capable of capturing uranyl in the entire practically interesting range of conditions (temperature and acidity) characteristic of liquid wastes containing uranium. Further improvement of the technology can make it economically feasible to extract uranium from sea water,” concluded Konstantin Katin.





